Undergrad introduced me to an interesting unification of math, medicine, and physics – biophysics. I’ve decided to spend my short month of ENT research exploring the electrophysiological properties of guinea pig cochlear outer hair cells (OHCs). 🙂
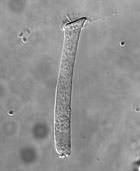
There are lots of things the PGY2 and I have to do before beginning the experiment: pulling pipettes, calibrating the instrumentation, getting all the solutions/containers prepared and labeled, etc. Our unofficial timer begins when we sacrifice the guinea pig(s) each day since the OHCs are only viable for 4-5 hours. We remove the temporal bone, dissect out the Christmas-tree shaped cochlea (guinea pigs have 4 turns whereas humans only have 2.5 turns in the cochlea), and throw it in a trypsin-based solution for digestion. After a few minutes, we plate the contents and wait another 20 minutes hoping that some OHCs will be isolated and fixed properly. To put things in perspective, in the course of one experiment, we’re really lucky if we can get five good OHCs.
When the plates are ready, we put one under the microscope (100x + oil immersion) and look everywhere for an OHC. Then we carefully guide the pipette (tens of microns at a time) till it’s positioned near the synaptic (basal) pole of the OHC. The crucial step is creating a “seal” between the pipette’s tip and the OHC’s membrane. This is done by monitoring the pipette tip’s resistance. The resistance will gradually increase until a certain threshold is reached and we can claim the OHC and pipette are electrically coupled (per the literature).

If we’re lucky enough to get an OHC that cooperates, we proceed to apply negative pressure to basically pull the membrane away from the cell while simultaneously jolting it. Ideally, we’re trying to poke a hole in the cell with the pipette still coupled to it. This provides us a direct view into the cell’s inner electrophysiology, and it’s at this time we can manipulate different parameters (voltage, current, resistance, etc.) and subsequently calculate things like charge density and capacitance.
I have to admit that as a lover of physics, mathematics, and the laboratory (things which are virtually nonexistent during the basic science curriculum), I was overcome with a geeky joy as I walked into the biophysics lab. Parafilm, Bunsen burners, fume hoods, micropipettes, volumetric flasks, shiny gadgets which look really expensive, electrodes, microscopes, heck, even those deionized water bottles… I’ve missed bench work so much from undergrad!! Okay, you can stop judging me now. 😛







have you ever thought about derm?
I honestly haven’t. From my current understanding of the field, there’s a lacking “procedural component” for my tastes. Plus, I’m not cool enough for derm. 😉
Wow! Messy lab. That would bug the crud out of me! I’m a sorta-neat freak. 🙂 It’s cool that you’re back in the lab. Hope you get some good research out of this rotation!