Most organ systems have some form of autoregulation to provide a steady supply of blood flow and oxygen to maintain normal physiologic function. This autoregulation is largely driven by chemical mediators to “couple” metabolic demand with blood supply. In other words, if the organ demands less oxygen, then it will be supplied with less blood. The brain and its associated vasculature are a perfect example of autoregulation in action!
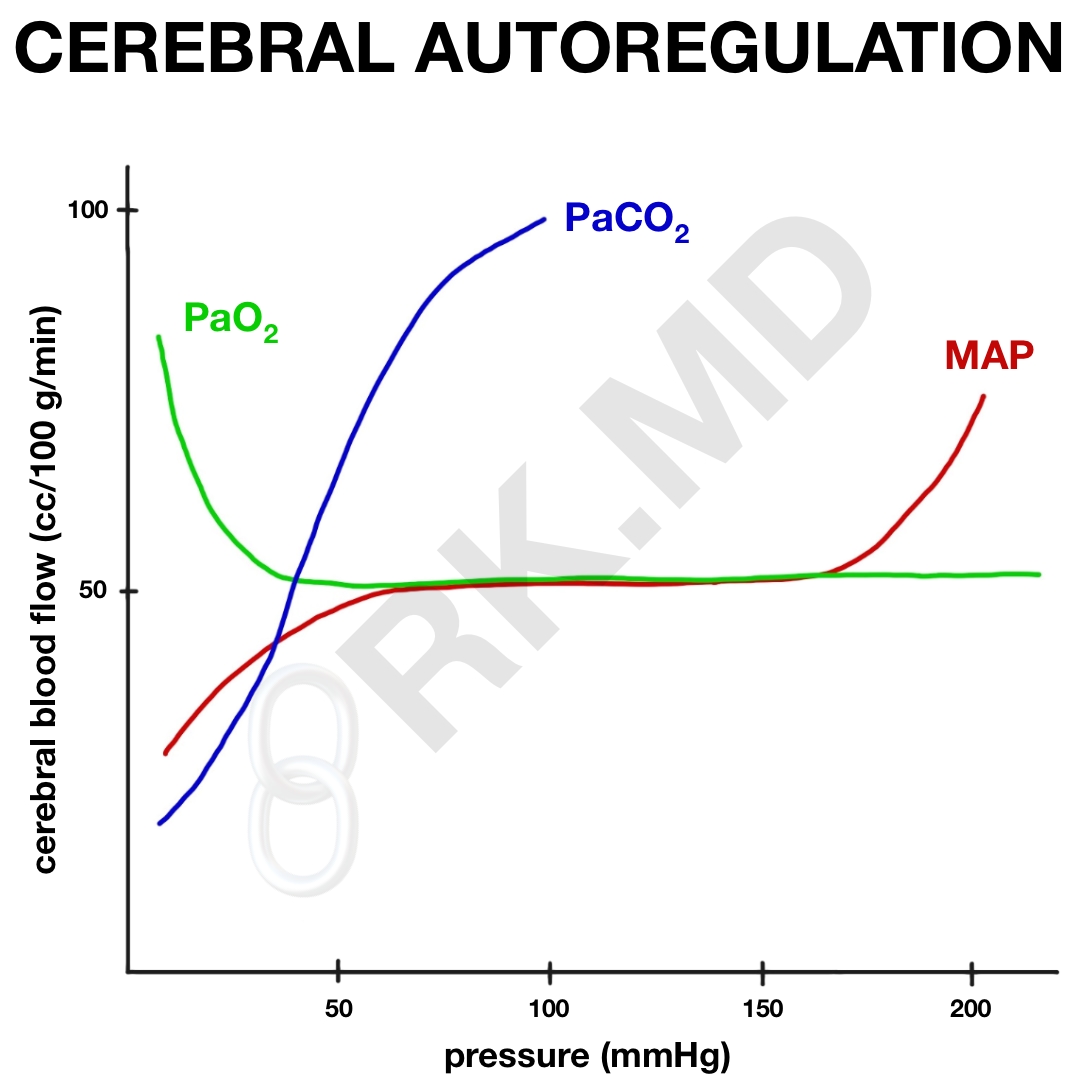
Cerebral perfusion pressure (CPP) is defined as the mean arterial pressure (MAP) minus the intracranial pressure (ICP), or CPP = MAP – ICP. If the central venous pressure (CVP) exceeds the ICP, then the CPP = MAP – CVP instead. Normally, the CPP generates a cerebral blood flow (CBF) of ~50 cc per 100 grams of brain tissue per minute (cc/100g/min). Therefore, high systemic MAPs can translate to high CPPs and vice versa. This can be catastrophic during hypo OR hypertensive episodes, but thanks to autoregulation, the brain does a remarkable job of maintaining constant CPP over a wide range of MAPs by the myogenic actions of vascular smooth muscle that constrict when MAPs are high (thereby “shielding” the brain from high blood pressure) and dilate when hypotensive (to send more blood to the brain). This autoregulation occurs primarily at MAPs of 60 – 160 mmHg, but in patients with chronic hypertension, may occur at an even higher range.
Additionally, the partial pressure of carbon dioxide (PaCO2) is incredibly important in cerebral autoregulation. Normally, our PaCO2 is ~ 40 mmHg. As this drops, the vasculature constricts dropping CBF by ~ 1-2 cc/100g/min for every 1 mmHg drop in PaCO2. By extrapolating this, you can see that during hyperventilation, a very low PaCO2 will significantly drop CBF. This can be useful in extenuating circumstances (ie, impending brain herniation), but also places the brain at a real risk for ischemia-related damage. On the contrary, as PaCO2 increases (ie, CO2 retention from narcotics), the cerebral vasculature will dilate leading to increased CBF which can be detrimental in patients struggling with preexisting elevations in ICP.
Interestingly, the partial pressure of oxygen (PaO2) really doesn’t affect CBF in HYPER-oxia. As the patient becomes more hypoxemic, CBF will start to increase once the PaO2 drops below ~40-50 mmHg to deliver more blood (and therefore oxygen content) to the brain.
Hopefully this illustrates the importance of MAP, PaCO2, and PaO2 on cerebral blood flow! Drop me a comment below with questions! 🙂






learning about CMRO2 and CBF, coupling and uncoupling, and I have a quick question..
So with the narcotics, benzos, barbs, propofol, etomidate, and dexmedetomidine, it’s said that the CMRO2 decreases, and the CBF decreases in parallel, and this is coupling. With the volatile anesthetics, this is uncoupled, and that’s because CBF increases due to vasodilation, while the CMRO2 decreases. So then, if CBF increases occur with vasodilation, CBF decreases occur due to vasoconstriction.
So then, if CBF decrease means vasoconstriction, does that imply that all of the above items cause cerebral vasoconstriction as CMRO2 decreases? So then I’ve heard of propofol as a systemic vasodilator – does it cerebrally vasoconstrict? Is there a distinction that some medications that vasodilate will do something else specifically in the brain? Thanks!
The propofol decreases CBF and CMRO2
and may also decrease the ICP particularly when combined
with hyperventilation.
The decrease in CBF with propofol
does not appear due to any direct vascular effects (unlike
pentothal) but due to a reduction in CMRO2
. It causes
a dose dependent but not necessarily an identical reduction
in MABP and ICP.
So glad you hit on this — so timely for my anesthesia exam. I have been so confused by the terms “coupled” and “uncoupled” however now after reading your explanation of coupling, I think I may understand these fancy terms. So, the oxygen & RBC are PAIRED/”coupled” — correct? So is this coupled means paired…. Uncoupling means unpaired, right?
So in appying this, here are my lecture notes:
– CBF-CMRO2 coupling
– changed in CMRO2 will result in similar changed to CBF.
– Volatile Anesthetics (sevo/iso/Des) cause uncoupling (unpairing of O2 & RBC??). VAs are potent cerebral vasodilators at >0.6-1.0 MAC. Can you explain this? Does the VA bump the O2 particle of the RBC? are they competitive? What exactly causes the “uncoupling”.
Thank you in advance for your spin on it 🙂
— Shelley in Philly
Don’t think about coupling in terms of RBC, oxygen, etc. Think about it in terms of the brain’s demand for oxygen (maintaining neuronal integrity, electrical activity, etc.) and the supply of oxygen provided by blood.
Arterial oxygen content = (1.34 x SaO2 x [Hb]) + (0.003 x PaO2).
The brain does an excellent job of increasing blood flow (CBF) as its metabolic need for oxygen (CMRO2) increases. It’s these two variables that are normally “coupled” – as one goes up, so does the other.
All halogenated volatile anesthetics above roughly 1.0 MAC will cause uncoupling BECAUSE they DECREASE neuronal activity (CMRO2) while INCREASING blood flow due to vasodilation. This leads to more blood than the brain actually needs resulting in “luxury perfusion” and a mismatch between oxygen demand and oxygen supply thereby “uncoupling” CMRO2 and CBF.
Hopefully this makes sense!
Yes – Very Helpful! The concepts I am clear on, it was just applying the terms “coupling/uncoupling” that was throwing me – as you have now cleared up. Thank you for helping break it down.
Happy to help! 🙂
Whoa! Concisely explained Dr. Rishi, now I know PaCO2 is even more important in maintaining normal CBF. And I thought for a moment, oh narcotics isn’t that bad since it makes us have more CBF?
Hahaha, I’m glad you enjoyed the read! 🙂
My pleasure sir, thank you for the knowledge you keep sharing, I really appreciate.