Extracorporeal membrane oxygenation (ECMO) is a form of mechanical circulatory support (MCS) used as a bridge to recovery, transplant, or alternative therapy (ie, VAD insertion). Whether it’s the actual cannulation process or day-to-day management, it’s one of my absolute favorite topics as an intensivist and cardiac anesthesiologist! ECMO’s usage is growing each year, and machines are becoming increasingly portable giving rise to therapies like ECPR. In this post, I’ll try to provide a simplified overview of this device.
Veno-venous (VV) ECMO is used for isolated respiratory failure (interstitial lung disease, flu ARDS, end-stage cystic fibrosis, etc.) where veno-arterial (VA) ECMO is used for combined cardiopulmonary failure (PE, refractory ventricular arrhythmia, cardiac arrest, MI, support for “high risk” percutaneous procedures, and more). In both VV and VA ECMO, blood is drained from a large vein, sent through a membrane oxygenator, and then pumped back to the body into an artery (VA) or a vein (VV).
COMPONENTS
The basic components in an ECMO circuit are the inflow/outflow cannulas, a pump, and a membrane oxygenator with heat exchanger where oxygen and carbon dioxide are exchanged. Modern oxygenators are lined with materials that resist thrombus formation; however we routinely look for fibrinous aggregates (platelets, red cells, etc.) collecting on the membrane which will increase resistance to flow. Compared to cardiopulmonary bypass (CPB), ECMO doesn’t require venous reservoirs, gravity drainage, nor circuit bridges. The modern pumps are preload and afterload sensitive, but can still generate flows of 0.5 to 7.0 liters/minute!
CANNULATION
Specifically with VA-ECMO, cannulation can be done centrally or peripherally.
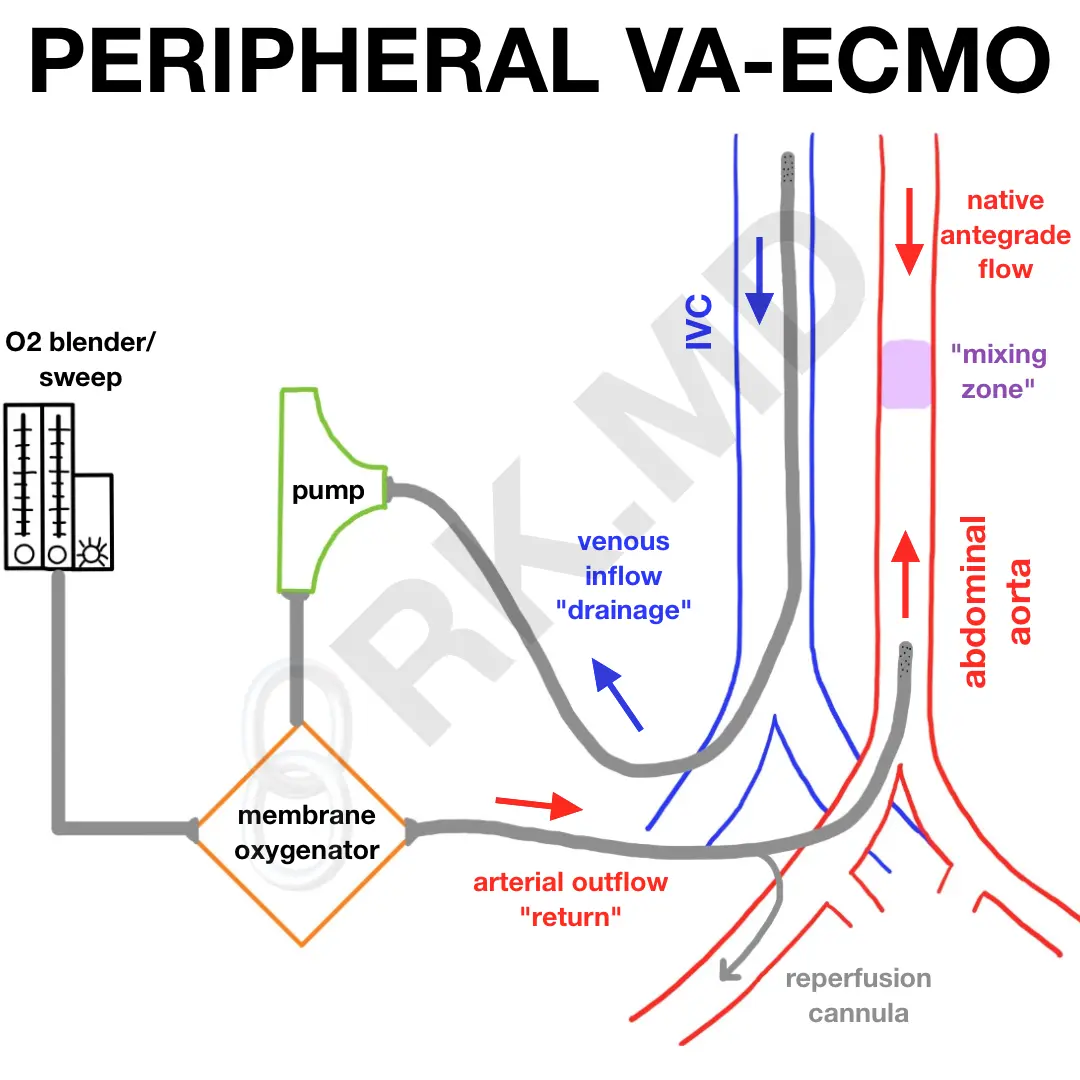
The central configuration is most often utilized after failed separation from cardiopulmonary bypass where venous and arterial cannulas are typically located very proximal (“central”) to the heart (ie, right atrium and ascending aorta, respectively). In peripheral VA-ECMO, virtually any large artery and vein can be used. I’ve most commonly seen the femoral artery and femoral vein (“fem-fem”) cannulated as shown in the diagram above. It’s important to understand that in this case, the femoral artery cannula is perfusing in a retrograde manner (ie, typically blood flows down to the body, not up into the aorta). Furthermore, because this cannula “fills” the femoral artery, distal perfusion to the leg can be compromised. A distal reperfusion cannula is connected to the femoral arterial cannula and often placed in the superficial femoral artery (SFA) to provide antegrade blood flow down the leg.
PRESSURES
In the diagram above, let’s define the following pressures:
- P1: venous drainage pressure from the IVC to the pump
- P2: pressure from the pump to the membrane oxygenator (“pre-membrane”)
- P3: pressure from the oxygenator to the arterial cannula (“post-membrane”)
An increase in P1 could suggest that there is impaired venous drainage (kinked or malpositioned venous cannula, hypovolemia, etc.) which is choking off fuel to the pump. An increase in both P2 and P3 could suggest increased afterload (arterial cannula kinking, increased vascular resistance, etc.) If P2 increases and P3 drops, perhaps there’s thrombus formation in the oxygenator.
CONSOLE OUTPUT
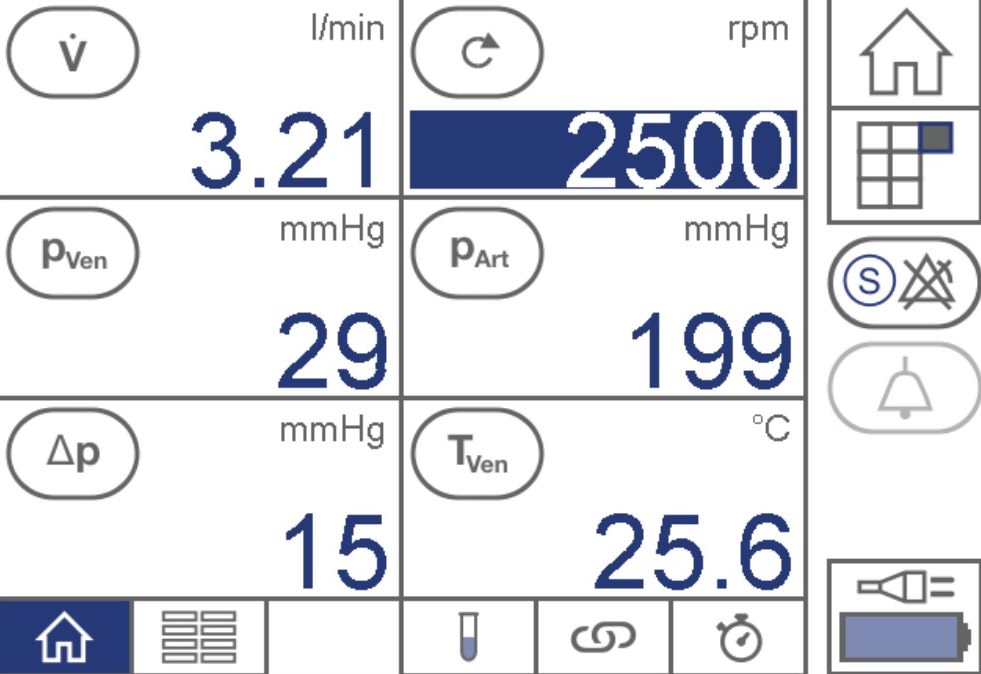
Reading the console is fairly straightforward. Machines like the CARDIOHELP can be operated in speed control or flow control modes where one directly controls either the pump’s speed or the overall flow rate, respectively. These parameters can change based on changes in circuit/vascular resistance. In the above example (speed control), we’re at 3.21 liters/minute. If the resistance goes up, flows will drop unless the pump speed is also increased (ie, 3000 rpm).
Δp refers to the pressure gradient across the oxygenator. If this increases, it too could suggest impending thrombosis necessitating a circuit change.
“NORTH-SOUTH” SYNDROME
Can’t talk about peripheral VA-ECMO without discussing the potential oxygen disparity that one may find in different parts of the body. This phenomenon has many names including “north-south syndrome”, “harlequin syndrome”, and “differential oxygenation.” In any case, if one understands the path of a red blood cell (RBC), they will also understand why north-south syndrome occurs, how to diagnose it, how to treat it, and how VA-ECMO really works.
One can be on VA-ECMO with varying levels of native cardiac and pulmonary function. Also, consider that the ECMO circuit cannot capture 100% of a patient’s blood content. Blood that isn’t drained will enter the right heart like normal, pass into the lungs, return to the left heart, and then get pumped out to the aorta by the left ventricle (LV). As the patient’s native heart function recovers, they will eject more blood DOWN the aorta which meets blood coming UP from the femoral arterial ECMO cannula. This results in an area of turbulence labeled the “mixing zone” in the diagram. There’s a “tug-of-war” between native cardiac function (antegrade flow) and ECMO pump inflow (retrograde flow) which dictates where this mixing zone ends up in the aorta.
If the lungs aren’t doing their job, the blood that is exiting the heart and perfusing organs proximal to the mixing zone (ie, the upper body) won’t be adequately oxygenated or ventilated compared to blood returning from the femoral artery distal to the mixing cloud (ie, visceral organs).
Also consider that the head and upper extremities drain into the superior vena cava (SVC). The lower body drains into the inferior vena cava (IVC) which is where most of femoral vein cannula drains its blood into the ECMO circuit. One can imagine that in north-south syndrome, the upper body’s oxygen delivery is already low because it’s derived from poorly oxygenated blood leaving the LV. This translates to even lower blood oxygenation in the SVC after tissue oxygen extraction. This severely deoxygenated blood returns to the heart, goes through lungs which don’t work, and exits the LV again to provide little oxygen to the upper body resulting in a vicious cycle of hypoxemia.
So how can we fix this? Consider if a multistage venous cannula was placed in the right internal jugular (IJ) vein to drain blood instead of the femoral vein. Now the blood that was poorly oxygenated in the upper body is being drained into the ECMO circuit for appropriate oxygenation/ventilation. Also, in addition to the femoral artery outflow, what if another outflow was placed into the internal jugular vein? This configuration, known as V-AV ECMO, drains blood from the femoral vein and infuses it back via the internal jugular vein AND femoral artery. Because the heart is receiving highly oxygenated blood from the internal jugular vein cannula, the patient’s pulmonary function doesn’t matter – oxygenated blood will leave the LV and provide adequate oxygen delivery to the upper body.
A more invasive alternative is to convert peripheral VA-ECMO to central VA-ECMO.
ANTICOAGULATION
Thrombosis and bleeding are two potential complications for patients on ECMO. Since blood will be in contact with the ECMO circuit, the coagulation cascade and a myriad of other pathways are activated. Without anticoagulation and appropriate flows, there’s a risk for thrombus formation on circuit elements which can embolize into the arterial system. Some circuits and oxygenators have special coating (heparin-bonded, PMP, etc.), but in general, we must remain vigilant for clot formation (visible clots, increased pressure gradient across the oxygenator, etc.)
Heparin, by far, remains the anticoagulant of choice for patients on ECMO. Sometimes we’ll use bivalirudin as an alternative, so patients don’t develop heparin-induced thrombocytopenia (HIT) while waiting for a potential transplant that requires cardiopulmonary bypass where heparin is preferred.
VENTING
To allow the LV to rest, it’s important that it stays empty to decrease wall tension and myocardial oxygen demand. Despite adequate venous drainage, some blood will always find its way back to the LV via pathways like aortic insufficiency and bronchial venous drainage. Devices like the Impella are used in conjunction with VA-ECMO (“Ecpella” configuration) to actively “vent” the LV.
CONTRAINDICATIONS
ECMO is a bridge to something. Whether that’s recovery or transplant, one must consider the potential repercussions of placing someone on this support device. Patients who have widespread metastatic cancer, an irreversible neurologic injury, contraindication to anticoagulation, aortic disruption, or are simply too frail should not receive this treatment.
Hopefully you found this (simplified) overview helpful! Drop me a comment below with questions! 🙂






This is a great resource, and makes a difficult topic simplified– thanks so much ! I’m just confused about one part:
“Consider if a multistage venous cannula was placed in the right internal jugular (IJ) vein to drain blood instead of the femoral vein. Now the blood that was poorly oxygenated in the upper body is being drained into the ECMO circuit for appropriate oxygenation/ventilation”
With V-AV ECMO- aren’t we placing a cannula into the RIJ for ECMO outflow? not to drain blood but to pump in oxygenated blood from the circuit?? This has me totally confused !!!
Thanks so much for the kind words, Katrina! Your second statement regarding V-AV ECMO is absolutely correct. What I was referring to in what you quoted is actually VV-A ECMO (two points of venous drainage: the femoral vein and the internal jugular vein, and a single point of arterial return: the femoral artery). This configuration can also offer effective oxygenation and hemodynamic support while minimizing the North-South syndrome I mentioned in the post. It’s just another alternative. 🙂
Hi Rishi! Been following you as well in IG… as always, your teachings are easy to understand. Thank you for all you do!!!
Can i also ask, i recently came across to a case of post open heart cabgx4 that deteriorates on day 3 was put in peripheral VA ecmo and was then inserted iabp as well. I noticed that when iabp was put on 1:2, the pt is augmenting well with better MAP. Is it because in 1:1 it mostly blocking the flow in each beat? Competing with theecmo flow?
How does this work ecmo with iabp.
Appreciate it in advance!!
Rishi,
Your education material is amazing! The residents must love working with you. Keep on doing what you do. I think that you are really making a difference in medical education!
Laura McNeill
Thanks so much for the kind words, Laura! 🙂
This is great! Thanks so much! Can you do something like this for other mechanical circulatory support devices (VADs, Impellas, IABPs, etc)?
Glad you found it helpful, Sarah! Check out my other mechanical circulatory support posts: link here. Additionally, feel free to use the search feature to find topics on LVAD, Impella, and IABP. 🙂