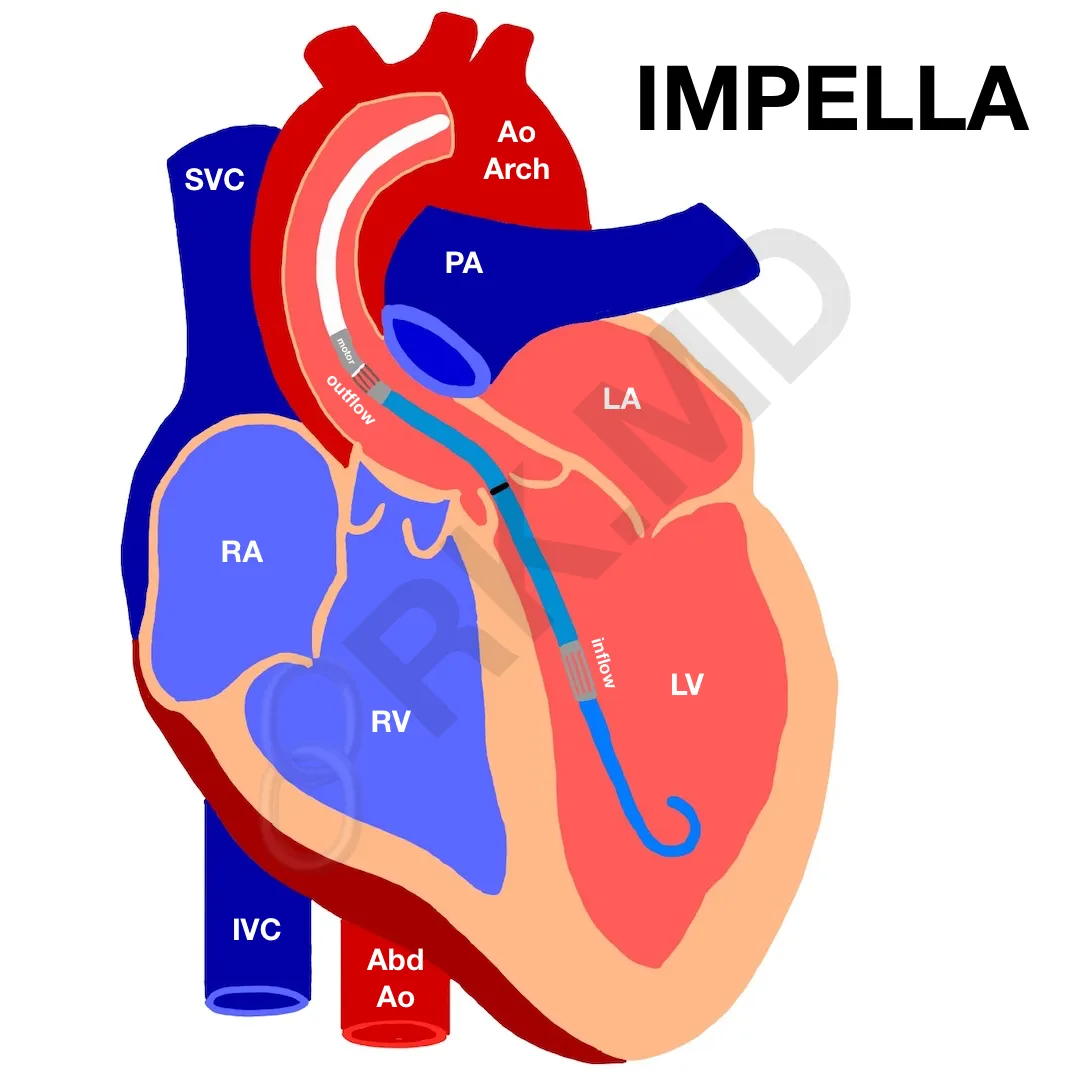
Impella® is a percutaneous axial heart pump placed retrograde across the aortic valve into the left ventricle (LV) utilizing fluoroscopy +/- echocardiography. Its indications include high-risk percutaneous coronary intervention (PCI, especially cardiogenic shock or signs of ongoing ischemia), post-cardiotomy failure, and myocarditis. In addition, it is routinely used as a bridge between other forms of circulatory support like intra-aortic balloon pumps (IABP), ventricular assist devices (VAD), artificial hearts, and extracorporeal membrane oxygenation (ECMO). In 2014, the (underpowered) RECOVER RIGHT study showed benefits with the Impella RP device to help those with right-sided heart failure refractory to medical therapy. For this post, I’ll only discuss the traditional left-sided Impella.
Without going into detail about the different power/rpm settings (P1 – P8), purge system, and other parameters, the intracardiac portion of the Impella consists of a pigtail to stabilize the catheter in the ventricle, an inflow where blood is suctioned from the LV, a radiopaque marker which is aligned with the aortic valve annulus under fluoroscopy, an outflow segment which propels blood into the ascending aorta, and a motor housing. The newer generation Impella 5.5 device does not have a pigtail, but instead, it advanced farther into the LV cavity (~5 cm versus 3.5 cm of previous generations).
This device accomplishes many hemodynamic goals:
- reduces left ventricular end-diastolic pressure (LVEDP) and volume (preload)
- reduces afterload
- reduces myocardial oxygen demand
- increases overall forward flow and mean arterial pressure (MAP)
- increases coronary perfusion
Impellas traditionally come in several flavors:
- Impella 2.5 (9 French catheter, 12 French motor) has a peak flow of 2.5 liters/minute and can be placed into the LV from the femoral artery.
- Impella CP (9 French catheter, 14 French motor) has a peak flow of 4.3 liters/minute and can also be placed from the femoral artery.
- Impella 5.0 (9 French catheter, 22 French motor) has a peak flow of 5.0 liters/minute but requires surgical placement (femoral artery cutdown, subclavian artery graft, etc.)
- Impella 5.5 has a peak flow of over 6 liters/minute and is placed surgically in the axillary artery.
In general, flow ~ [rpm (power) / (aortic diastolic pressure – LV diastolic pressure)]. Therefore, the sicker the left ventricle, the smaller the difference between the aortic and left ventricular diastolic pressures, and therefore, the higher the flow for a given power setting.
Sometimes we’ll use Impella devices as ventricular vents for patients on ECMO for many weeks. In other situations, patients will have them for longer than 4-6 days as their native heart function slowly recovers.
Placing these devices can lead to vascular injury, bleeding (from placement and because we need to heparinize), intracardiac injury (especially of the aortic valve and papillary muscles). In addition, the axial pump can “chew up” cells at higher speeds leading to some degree of hemolysis and thrombocytopenia. Additionally, microemboli and plaques can be dislodged leading to renal dysfunction, strokes, etc.
With this in mind, a few contraindications to Impella placement include a mechanical aortic valve, significant aortic stenosis/insufficiency, LV thrombus/rupture, cardiac tamponade, and severe peripheral artery disease.
I’m definitely a fan of axillary Impellas since patients can still ambulate, but femoral Impellas are very finicky regarding positioning. Because the inflow and outflow segments have to be properly positioned, if the patient moves their hip even slightly, they may move the catheter tip resulting in decreased cardiac output and suction events along the ventricular wall, valve, or chordae.
Drop me a comment below with questions! 🙂


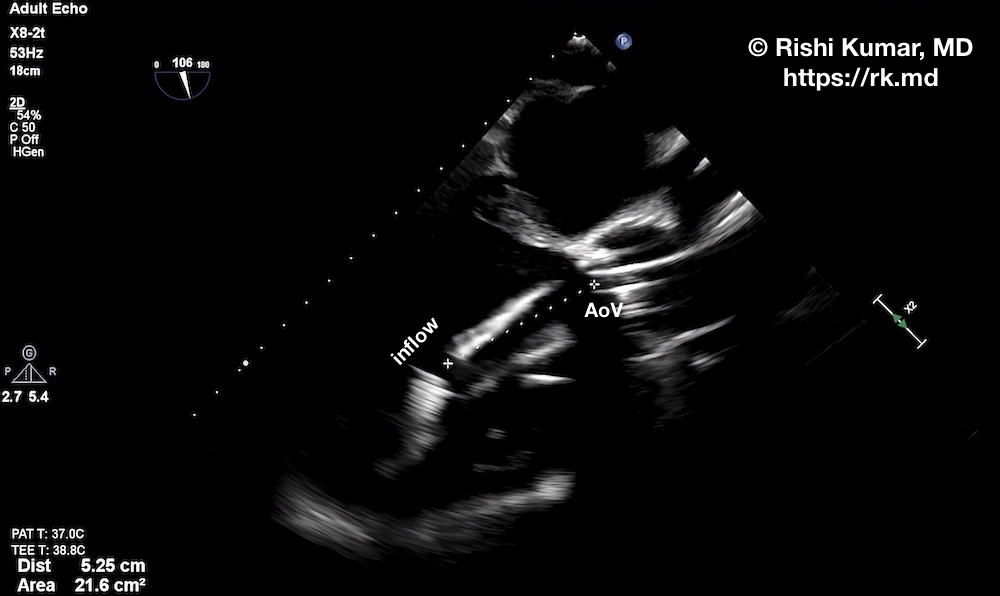




Hi I was wondering if you could discuss more on the topic of Impella placement for ECMO venting as far as the criteria patients need to meet for this additional support?
Peripheral VA-ECMO aims to establish perfusion back to the body, but at the expense of increasing LV afterload due to retrograde flow. This can be even worse in patients with aortic valve insufficiency (the LV will get flooded) or poor venous drainage by the ECMO, but I’ve seen vents routinely placed in most patients. The Impella’s purpose is to offload (“vent”) the LV. This, in turn, decreases wall stress and myocardial oxygen demand allowing the heart to rest and ideally recover. Data surrounding this strategy seems to show mortality benefit to venting the LV, but doesn’t seem to show a major difference between a surgical LV vent (typically placed through the right superior pulmonary vein) or an Impella.
May I use your image of the impella for a talk at my hospital? Not commercial.
Hey Kelley! Yes absolutely – just please give credit. Thanks for letting me know! I hope it supplements your talk! 🙂
Do you place these (impella 2.5 or CP) yourself as an CCM and CVA specialist or rely on a cardiologist or surgeon?
Nah my surgical/cardiology colleagues place them.
Always learn something, and so interesting Rishi. Thanks?
Absolutely, Sharon! Thank you so much! 🙂
So happy I found you, and this post specifically! I’m a tech new to the CVICU (and pre-PA student) and there is so much to learn about all the devices I see on our unit. I was able to watch an impella removed at bedside today so I decided to read up on them a bit more. Thanks for the info!!
Thanks so much for the comment, Charlie! Glad you found this post useful! Good luck on your PA journey!
Do you feel balloon pumps falling out of favor for treatment of carciogenic shock?
I don’t think balloon pumps will fall out entirely quite yet due to the ease of their placement and literature in the last five years showing no significant difference in major adverse outcomes in the short-term (~1 month) time frame (ie, PROTECT 2 trial). The cost difference in the devices is astronomical, but one could argue that in the long term, the true costs are equatable. Time will tell. 🙂
Could you comment further on the use of impella for LV vent while on (presumably) VA ECMO?
So there are several reasons for the LV to be overloaded during V-A ECMO like afterload alterations, aortic insufficiency, collateral bronchial flow, and right heart recovery. Inadequate LV unloading is a fairly common reason for failed ventricular recovery during ECMO. Impella offers one (very expensive) solution to empty the LV.