Left ventricular outflow tract (LVOT) obstruction is a potentially catastrophic etiology for hypotension due to several etiologies: idiopathic hypertrophic subaortic stenosis (IHSS), hypertrophic obstructive cardiomyopathy (HOCM), systolic anterior motion (SAM) of the mitral valve, and even mid-cavity obstructive hypertrophic cardiomyopathy. These obstructions can be dynamic in nature and consequently vary based on cardiac parameters like preload, afterload, contractility, heart rate, etc.
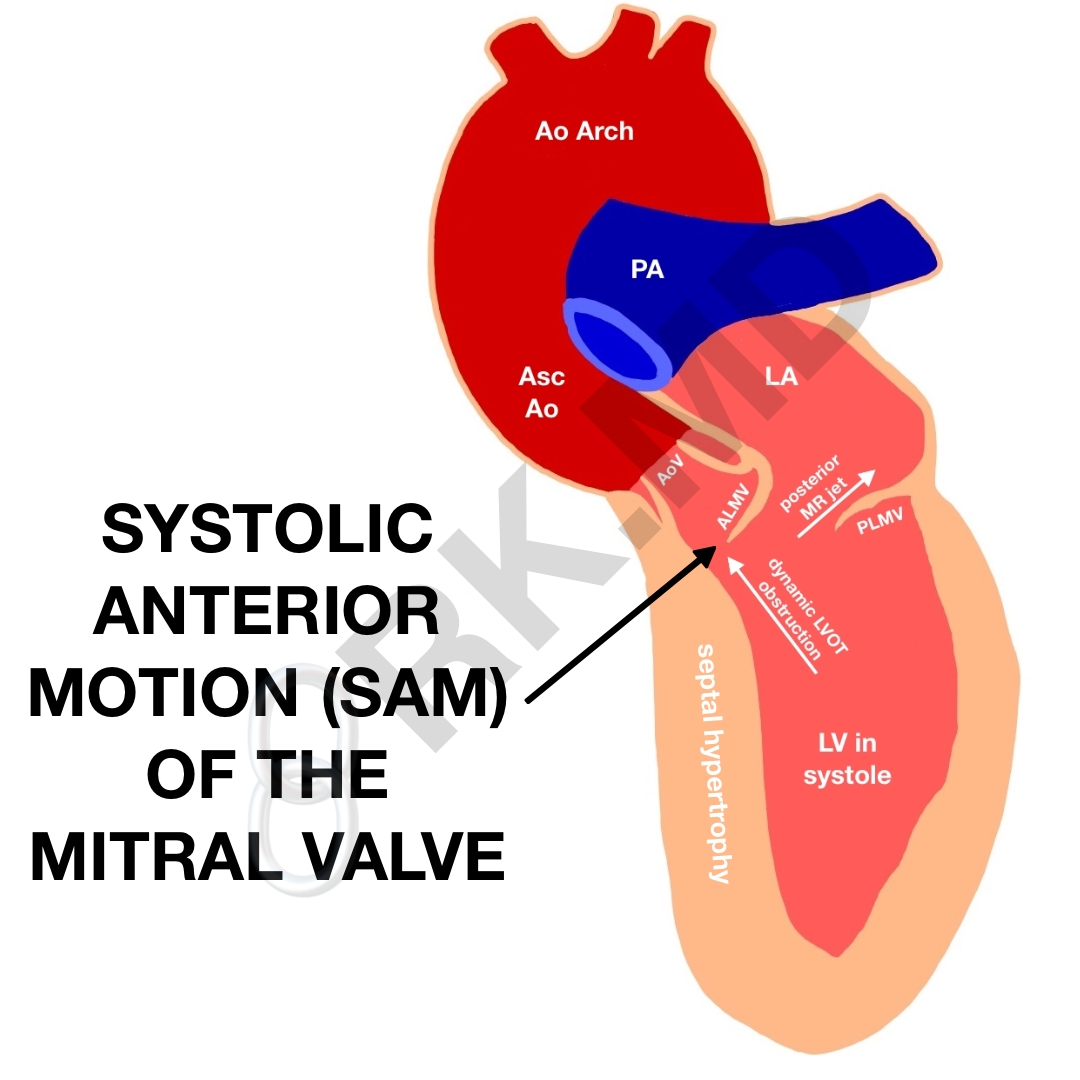
Abbreviations: systolic anterior motion (SAM) of the mitral valve, pulmonary artery (PA), left atrium (LA), left ventricle (LV), ALMV/PLMV (anterior and posterior leaflet of the mitral valve, respectively), aortic valve (AV), left ventricular outflow tract (LVOT)
For a specific example, let’s look at systolic anterior motion (SAM) of the mitral valve causing a dynamic LVOT obstruction. Risk factors for this process include a narrowed LVOT diameter from a thick intraventricular septum (IVS), an elongated anterior mitral valve leaflet which gets “sucked” into the LVOT with high blood velocities during systole (Venturi effect), and papillary muscles positioned more apically moving the mitral plane towards the IVS. In LVOT obstructions from any of the aforementioned etiologies, we have several hemodynamic goals to reduce the obstruction and improve forward flow.
In general, we aim to increase ventricular volume (“preload“) and reduce contractility. We can accomplish this by administering fluids, elevating the legs to increase venous return, and using agents like beta blockers for the dual benefit of negative inotropy and negative chronotropy. By keeping the heart rate on the low-normal side, we prolong diastole and allow the heart to fill more to alleviate the LVOT obstruction.
Here are some transesophageal echo (TEE) clips I acquired of SAM. You can see how the anterior leaflet of the mitral valve (ALMV) moves into the LVOT causing a dynamic obstruction. Furthermore, the classic finding of aortic valve (AoV) leaflet fluttering is also present.
Now consider this example around the time of inducing general anesthesia. We routinely administer medications like succinylcholine, nondepolarizing neuromuscular blockers, and antibiotics which can cause anaphylaxis at the beginning of surgery. How does anaphylaxis present in a patient under general anesthesia? Typically hypotension and tachycardia. How does a dynamic LVOT obstruction present? Typically hypotension and tachycardia.
Okay, but why does that matter?
Epinephrine is the mainstay treatment for true anaphylactic reactions to stabilize mast cell degranulation and support hemodynamics. However, epinephrine will drastically increase a patient’s contractility and heart rate… a bad idea in dynamic LVOT obstruction. The presentations are the same. The treatments are different. This is the benefit of bedside echocardiography to differentiate between the two! 🙂
Drop me a comment below with questions! 🙂


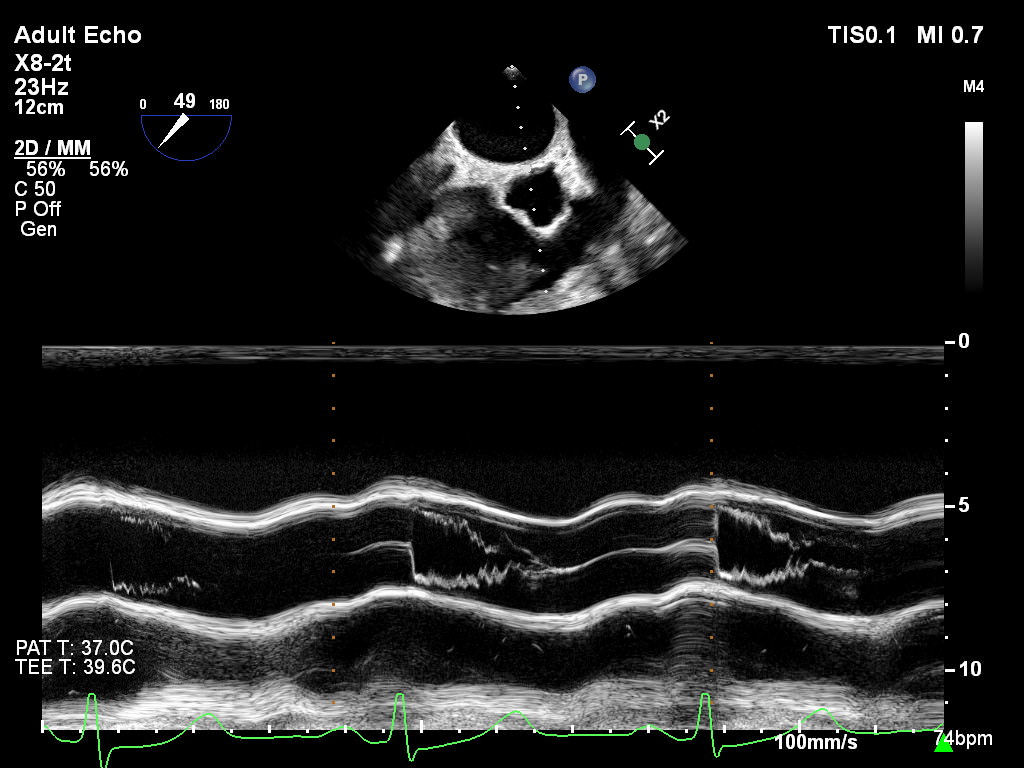




When evaluating the anterior mitral valve and SAM, not every instance results in LVOT obstruction. How do we know for sure that there is an LVOT obstruction? Is the only way to observe the dagger sign? What else can we look for to diagnose obstruction? Is valve replacement an option if the SAM is severe enough?
Yes, the “dagger” shape on spectral doppler interrogation of the LVOT does suggest a dynamic outflow obstruction. A lot of SAM we see isn’t clinically significant (ie, chordal SAM), and there are various levels of severity. 3D TEE of the aortic valve apparatus (LVOT – valve – ascending aorta) cropped down to the level of outflow can sometimes show the anterior mitral leaflet obstructing the outflow too.
We initially treat SAM with medical therapy. Beta-1 receptor blockade (reducing contractility and increasing ventricular filling) coupled with volume loading is typically attempted first. Surgery is usually a last resort, and many techniques are summarized in Table 1 of this article. Essentially, the surgical goal would be to move the mitral leaflet coaptation point more posteriorly and/or increase the distance between the coaptation point and the interventricular septum (Csept).
My reading for SAM was 1.8 m/ sec and now 4.6 m/sec what does that mean
They’re velocities, but I’m not sure of what. Ask the physician who ordered/interpreted the study.
Ok. So I looked at this last night but I wasn’t sure what you were asking. When I first looked, I thought Cardiomyopathy, and Mitral Valve Prolapse seemed to obvious to me. But, bc of the extent of the disease process, bad things can happen. So, I believe that Pulmonary Hypertension is already an issue. I see what you wrote about treating the Mitral Valve. So, my question is, How do you treat the patients who have progressed to pulmonary htpertension along with the other symptoms in the short term? What can we offer them for a comfortable, quality of life after the crisis? I also wanted to say that I’ve experienced Anaphylaxis response 3 times. SO very scary. Once to PCN, once to a Cephalosporin, and once to Peanuts. All allergies developed as an adult. Always carry an Epi-Pen, an inhaler, and Benadryl. Have chewed Benadryl many times to slow down the effects. Another question totally out there, but interesting. Have you ever had a patient with Malignant Hyperthermia yet? My nephew had it, really scary stuff! Was scared when my kids were put to sleep the first time. Thank you Rishi for this lesson. Look forward to ypur response?
So remember this isn’t a true mitral valve prolapse. This is systolic anterior motion (SAM) of the leaflet intermittently causing obstruction of the left ventricular outflow tract (LVOT). In general, for any degree of chronic mitral regurgitation leading to pulmonary hypertension, one must focus on the pulmonary vascular resistance (PVR), volume status, and right heart metrics. Maneuvers to decrease PVR (avoiding hypoxia and hypercarbia, using meds like epinephrine and milrinone, considering inhaled Flolan), optimizing volume status (usually keeping the patient a little on the dry side with diuresis), and supporting the right heart (optimizing volume, epinephrine and milrinone) can all help. There are better long term options for pulmonary hypertension like home oxygen, sildenafil, Bosentan, etc. Sometimes a mitral valve replacement is the best option, but we try to optimize patients the best we can before surgery.
That’s scary about anaphylaxis, but I’m glad you’re taking the necessary precautions!
I’ve never had a patient with malignant hyperthermia (MH), but it’s definitely a high-yield topic in the world of anesthesiology. Please make sure that this history is relayed for all future anesthetics, and if your nephew has any siblings, perhaps they should be aware of the potential for MH too.
I get what you’re saying now. I’m alwsys thinking treat with a surgically first. That’s not always best, or posible. Patient’s not always healthy enough to have surgery first. When treating with the right meds, and O2, and then if needed, surgery. Thank you Rishi?
Beautiful analogy with anaphylaxis I love that method of teaching! Thanks Doc!
Appreciate it, Ahmad! 🙂