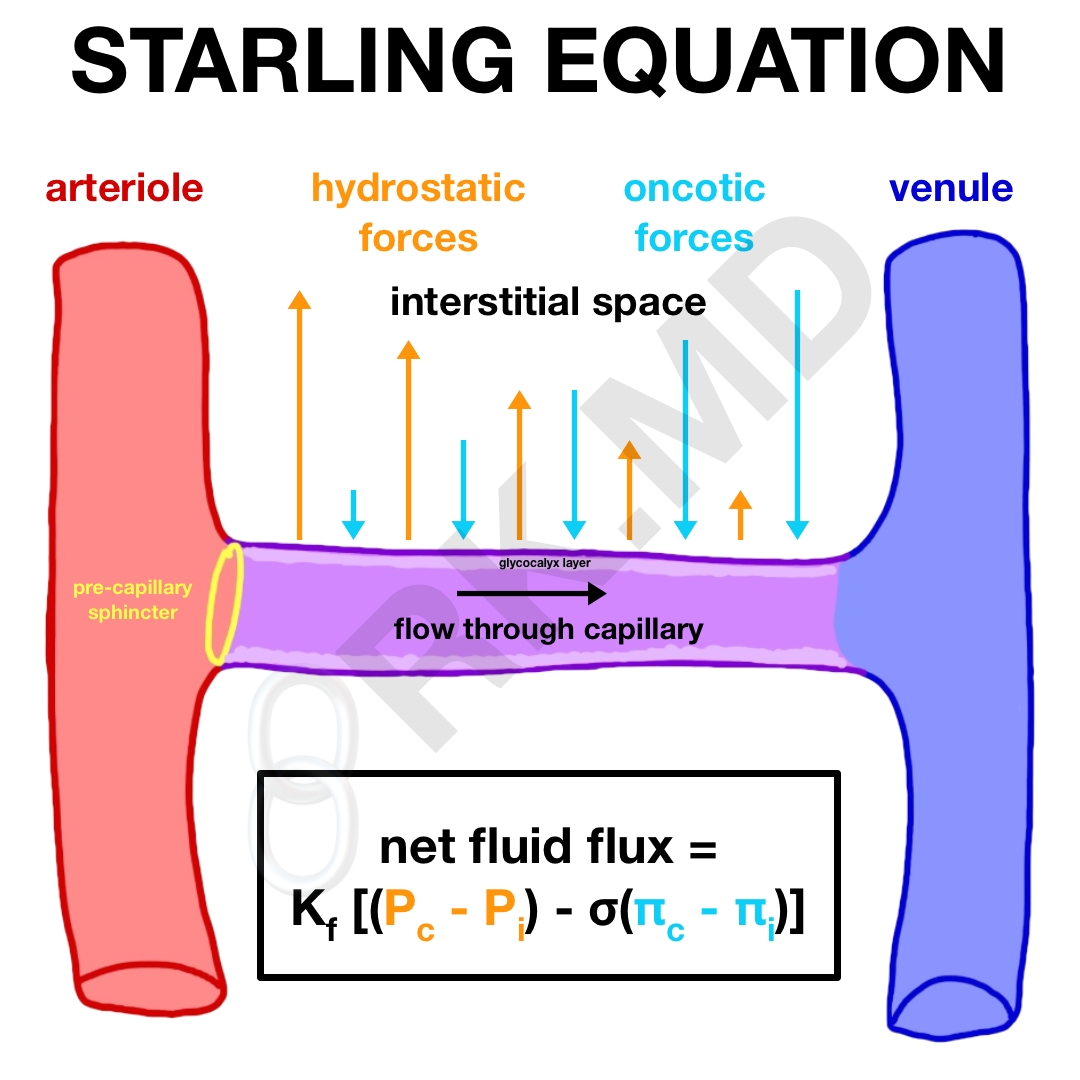
“Starling forces” describe the movement across capillaries as a balance between hydrostatic and oncotic pressure gradients within the capillary and surrounding interstitium. Filtration across the capillary membrane occurs along its entire length and is based on a net driving pressure gradient.
In the equation above:
- Kf represents the filtration coefficient – a product of the capillary wall area where flux can occur and the permeability of the wall-to-water flux
- Pc and Pi are the hydrostatic pressures in the capillary and interstitial space, respectively
- σ is the reflection coefficient which describes the capillary’s permeability to proteins. A coefficient of ‘1’ means the capillary is impermeable to protein whereas a coefficient of ‘0’ is completely permeable.
- πc and πi are the oncotic pressures in the capillary and interstitial space, respectively
The hydrostatic pressure is a factor of systemic perfusion of the capillary, the arterial and venous pressures, and the change in resistance across the capillary. Oncotic pressure is due to plasma proteins (namely albumin) that do not readily cross the capillary membrane. These proteins generate a “draw” of fluid back into the capillaries although we must keep in mind that the interstitial oncotic pressure (πi) can increase in conditions like “capillary leak” and myxedema thereby decreasing the protein concentration gradient between the capillary and interstitial compartments.
From the arterial to the venous end, there is a decrease in the hydrostatic pressure (causing fluid filtration) and an increase in the oncotic pressure (reabsorption). Oncotic pressure increases across the capillary length because as fluid leaves the capillary from the arterial end (due to Pc), the capillary protein concentration is effectively increased (πc). Generally, there is a net positive fluid flux out of the capillary into the interstitial space. This extra fluid outside the blood vessels is usually resorbed by the lymphatic system and ultimately drained back into the venous system. Edema becomes clinically apparent when the interstitial compartment gains 2-3 liters of extra fluid without sufficient drainage.
Another important thing to remember is that a pre-capillary sphincter maintains a form of pressure autoregulation to the arterial end of the capillary bed. In other words, with an increase in systemic blood pressure, the capillaries are exposed to a fairly constant perfusion pressure due to contraction and relaxation of this sphincter. The venous end of the capillary network does not have such tight regulation and is susceptible to additional fluid extravasation in conditions of venous congestion (volume overload, venous thrombosis, etc.)
Drop me a comment below with questions! 🙂






Are pre-capillary sphincters everywhere in the body? Why then the emphasis on arteriole control in blood flow in some books. Nice write up!
Hey Miguel! I’m honestly not sure. If I had to guess… no. Just like not all veins have valves for various reasons.
if you increase hydrostatic pressure, do you automatically decrease oncotic pressure?
Not necessarily! They’re generated by two different things. Hydrostatic pressure by cardiac contractility causing blood flow; oncotic pressure by a osmotic gradient (primarily from albumin).