The oxyhemoglobin dissociation curve (ODC) is one of the most recognized teachings of basic physiology. It describes the relationship between the saturation of hemoglobin and the partial pressure of arterial oxygen. Intuitively, it makes sense that the more oxygen available (a higher PO2), the more saturated hemoglobin will be (% saturation). But what if the hemoglobin is in a different conformational state because of acidosis or hemoglobinopathy? And once the hemoglobin molecule is saturated with oxygen, how readily will it “give up” the oxygen to end organs and tissues that require it?
Let’s start with the basics. Most adult hemoglobin is hemoglobin A (HbA), an iron-based metalloprotein made of two alpha and two beta globular protein subunits (a tetramer). Each HbA molecule can hold up to four oxygen molecules, but the hemoglobin molecule is also involved in physiologic buffering and carbon dioxide transport (carbaminohemoglobin).
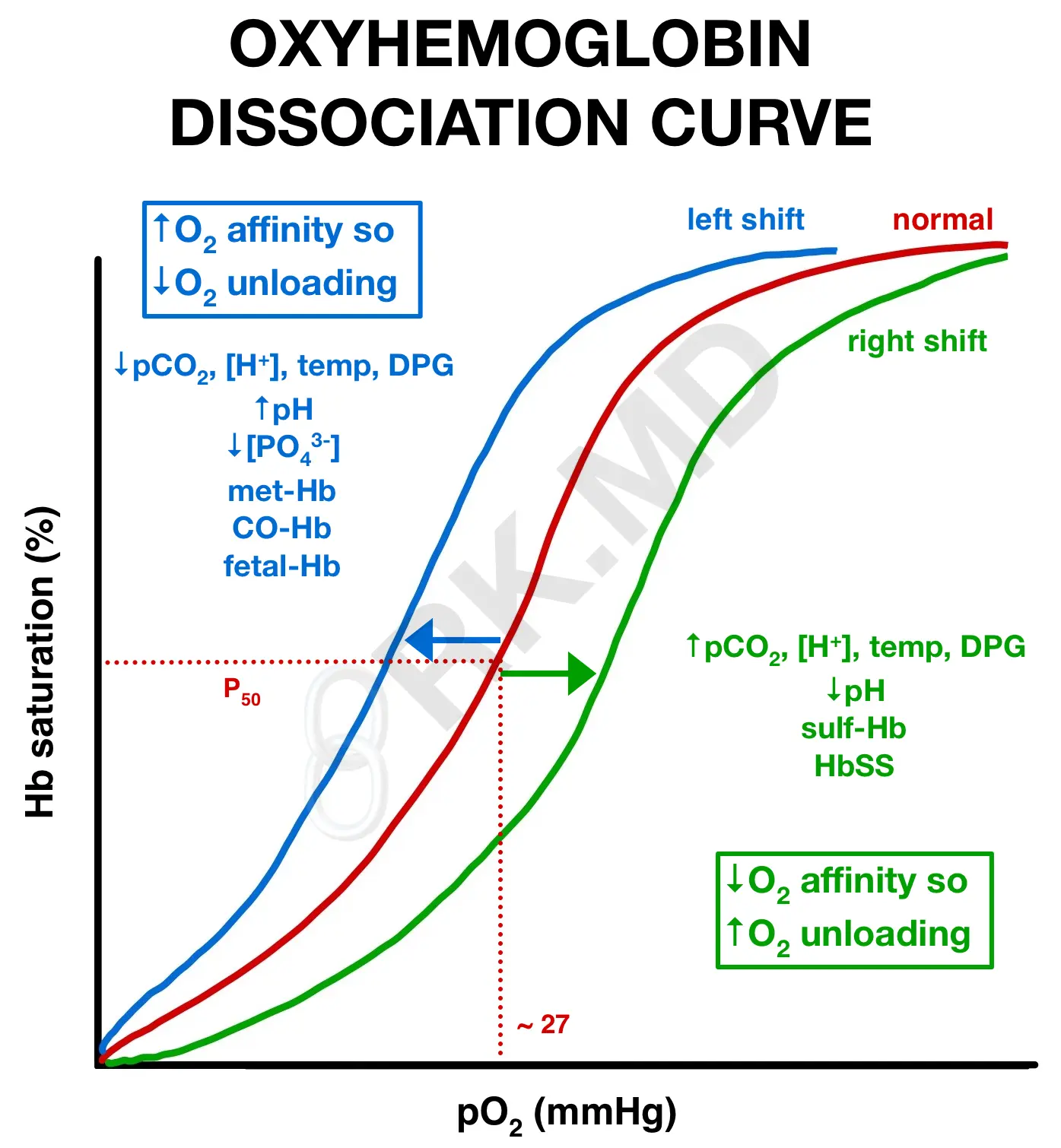
An important teaching point is cooperativity, where when an atom of oxygen binds to hemoglobin, the remaining unoccupied spots on that hemoglobin molecule have an increased affinity for oxygen. In other words, with each oxygen molecule, hemoglobin gets hungrier and hungrier for the next. This positive cooperativity is responsible for the ODC’s sigmoidal shape.
A PO2 of ~27 mmHg in healthy adults corresponds to ~50% hemoglobin saturation (red curve). This is known as the P50 of hemoglobin. Many physiologic stressors can shift the curve rightward or leftward, changing hemoglobin’s P50. It’s important to know what these are and what they mean.
A rightward shift of the ODC (green curve) means that a higher PO2 is required to achieve a similar hemoglobin saturation level compared to the baseline (red curve). This also means that the hemoglobin molecule has LESS affinity for oxygen and is MORE willing to unload oxygen at the tissue level. When considering factors that create a rightward shift, think of a warm exercising muscle with an increased temperature, increased CO2 production (and therefore decreased pH leading to an acidosis), and increased 2,3-diphosphoglycerate (DPG). DPG is a red blood cell metabolite of glycolysis that stabilizes the low-oxygen affinity form of hemoglobin (‘T’) and increases during hypoxia (think high altitude or exercise) and anemia. In these situations, hemoglobin needs to unload oxygen to the starved tissues. Also, sickle cell hemoglobin (HbSS) and sulfhemoglobin cannot readily bind oxygen (low affinity) and are right-shifted.
A leftward shift of the ODC (blue curve) means less PO2 can achieve a higher hemoglobin saturation than the baseline. Hemoglobin has a HIGHER affinity for oxygen and is LESS willing to give up oxygen molecules to peripheral tissues. Many factors that create left shifts (decreased temperature, alkalosis, decreased CO2, decreased DPG) are the opposite of those creating right shifts. Additionally, methemoglobinemia (metHb), a state where the iron moiety in hemoglobin is oxidized to the 3+ state and unable to accept oxygen like the typical 2+ oxidation state, creates a leftward shift. The portion of overall hemoglobin which is metHb has essentially removed binding spots for oxygen. Therefore, oxygen will bind more tightly to the areas it DOES have (higher affinity, left shift). The normal hemoglobin might be fully saturated, but it’s unwilling to give up the oxygen to peripheral tissues, so these patients can appear cyanotic. Similarly, carbon monoxide binds hemoglobin ~250x more readily than oxygen, reducing binding spots for oxygen and shifting the curve leftward. Fetal hemoglobin is structurally different than adult hemoglobin and adapted to have a high affinity for oxygen since the uteroplacental circulation has relatively low partial pressures of oxygen.
This is a fairly simplified overview of the ODC, so drop me a line if you have questions in the comments section below! 🙂






Hi! A little confused at this point ‘DPG is a red blood cell metabolite of glycolysis that stabilizes the low-oxygen affinity form of hemoglobin (‘T’) and increases during hypoxia (think high altitude or exercise) and anemia.’
I thought during hypoxia, the hemoglobin had higher affinity for oxygen, so why would DPG increase during hypoxia?
Thanks!
Hb job is to deliver O2 to peripheral tissues, during hypoxia body tissues are deprived of oxygen, so the hb affinity for oxygen decreases to easily provide O2 to tissues.
If affinity is high for O2 then it will not easily give O2 to tissues
that’s the easiest explanation i have found on this topic, amazing job
Thank you!
thank you for the article Dr Rishi .i greatly apreciate for it has helped me prepare for my upcoming exam .Keep on doing the great job.
Thanks for the kind comment! Glad you found it helpful!
Hi, thank you for sharing. I was looking for a nice Hb curve to include in a lecture for co-workers in a ward. The one you present along with a clear explanation would suit very well for the purpose. Do you mind if I use it in my slides? Not commercial. Best greetings and good luck in your job
hey, can i use the graph with credits given on free educational website for a teaching purpose?
Sure, just please give credit! Thanks for asking! 🙂
Great explanation
Thank you! 🙂
I am taking a chance that you are still monitoring this post.
This is my theory but I can’t seem to find it in the literature:
It would seem to me that a left shift in the ODC would result in a misleadingly high SpO2 (from a pulse oximeter) because hemoglobin would remain saturated even if there is tissue hypoxia. An increased cardiac output that is rapidly replenishing the PaO2 would keep the SpO2 reading higher because the bound oxygen cannot dissociate at that level of PaO2. However, if the heart rate decreases, the PaO2 would fall enough for the oxygen to dissociate and SpO2 would fall. An example of this would be increased endogenous production of CO in some forms of sepsis and other oxidative stress conditions. CO results in carboxyhemoglobin (not recognized by most pulse oximetry) AND shifts the ODC to the left. You would need co-oximetry to get an accurate SaO2.
I want to make sure I’m understanding this… so if there is a right shift would the SvO2 be lower and a left shift would give a higher SvO2 reading? Thank you in advance!
Hey Courtney, it depends on the partial pressure of oxygen in the blood (PO2). Based on the axes of the figure, to maintain the same hemoglobin oxygen saturation (SpO2), a “right-shifted” curve would need MORE oxygen and a “left-shifted” curve would need LESS. Remember, when describing the right/left shifts, it’s with regard to the x-axis (partial pressure of oxygen).
I have been taught in school that left shifts are generally better for the patient vs right shifts.
They can both have physiologic consequences.
Dr. Rishi,
Thank you for this post!! I am currently writing my capstone for my NP degree and was looking up refreshers on the oxyhemoglobin dissociation curve! Great explanation and diagram! Very helpful!
Happy you found it useful, Taylor! 🙂
Hi Dr. Rishi,
I love your explanation here of the Oxyhemoglobin Dissociation Curve!!
I will be referencing it for my students in a 3rd year physiology course at Bishop’s University.
Thanks!
Andrew Darnel, PhD
Thanks for the kind reference, Andrew! I’m all about freely spreading education!
Great overview of the topic! I am in my 3rd year of CRNA school and this was a nice refresher, particularly your coverage of some of the pathophysiology. I appreciate the time you took to put into this!
Thank you so much! 🙂
Your explanation of this concept makes so much sense. I’ve got my pulmonology final on Monday, at least I know I’ll get any questions related to the Hgb-dissociation curve right!
I really appreciate the kind words, Justin! Best of luck to you!
I’m an M2 currently studying for step 1 and I think that might have been one of the best and most concise explainations of that concept which is fantastic because I struggle with it. I’m pretty sure reading this article was the first time I actually understood the methemoglobin left shift where previously it was just a fact I needed to memorize. Thanks for the great teaching points!
Thanks so much for the thoughtful comment, Catherine! I’m glad I was able to help! Best of luck studying for Step 1! 🙂