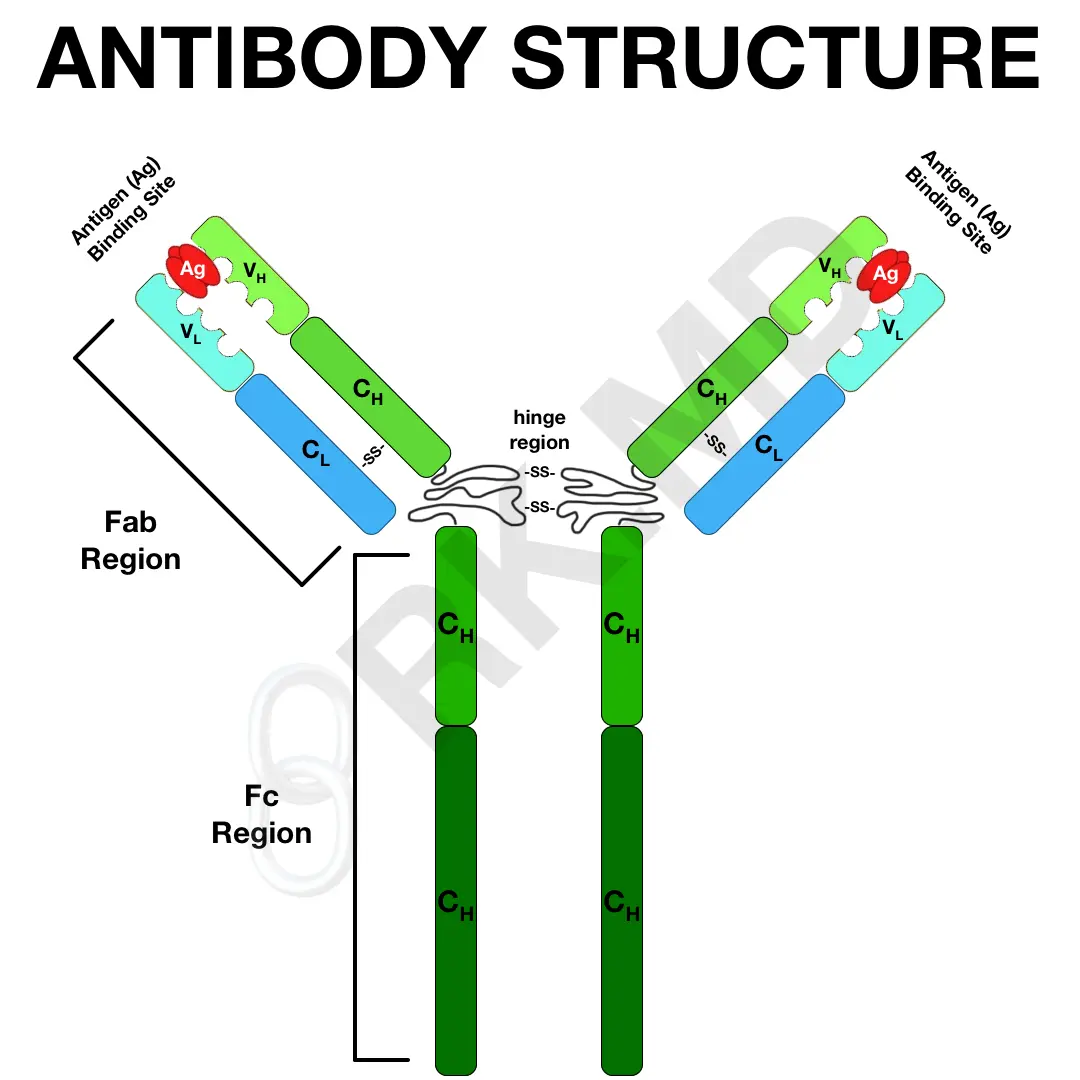
With all the discussion surrounding antibody production after receiving a COVID-19 vaccine, let’s talk about the structure of an antibody.
An antibody (immunoglobulin, or Ig) is a protein synthesized by plasma B cells targeting unique molecular structures called antigens (Ag) classically from bacteria and viruses (e.g., regions on the SARS-CoV-2 spike protein). When the immune system detects these antigens, a series of adaptive response events generate antibodies targeting them for destruction.
The fragment crystallizable (Fc) region comprises two heavy chains (H) that modulate immune system activity by interacting with surface receptors, complement, etc. These heavy chains also determine the antibody class:
- IgA (10-15%, dimer): GI tract, mucosal surfaces, breast milk – primary defense against inhaled/ingested pathogens
- IgD (< 1%, monomer): modulates humoral response, unclear role
- IgE (least prevalent, monomer): increases in allergic conditions, binds mast cells
- IgM (5%, pentamer): synthesized first in response to pathogen
- IgG (70-75%, monomer): most abundant, involved with neutralization of pathogens, crosses the placenta
The fragment antigen-binding (Fab) regions bind to antigens. Each consists of a heavy chain (H) and light chain (L, either kappa or lambda). The variable (V) domain of the H and L chains creates the paratope, which binds an antigen at the antigen-binding site.
In summary, an antibody consists of two heavy (H) and two light (L) chains. These are spread across constant (C) and variable (V) domains. Disulfide bonds (-SS-) hold parts of the antibody together.